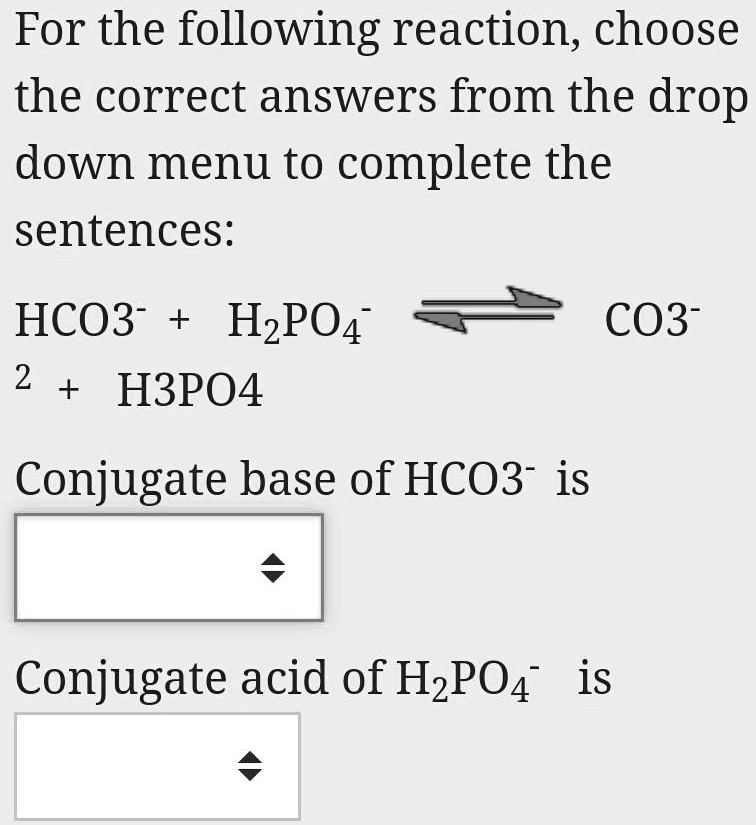
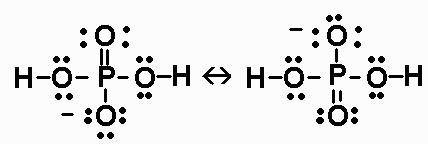15) Circle one and tell whya. CH3COOH is a n) (acid, base, salt)b. NH4Cl is a(n) (acid, base, salt)c. KOH - Brainly.com
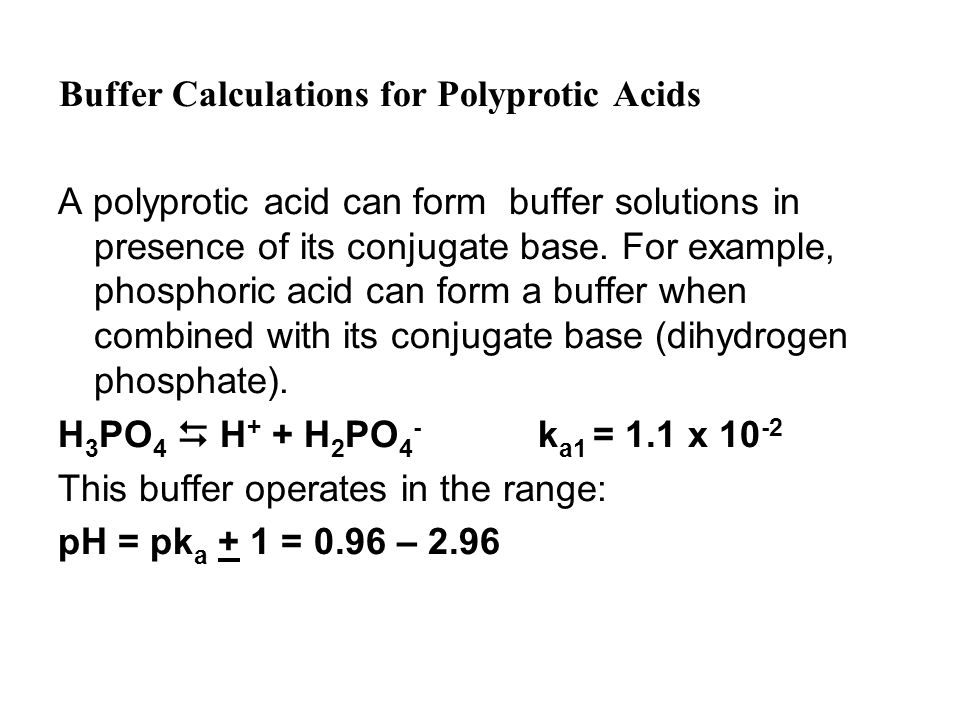
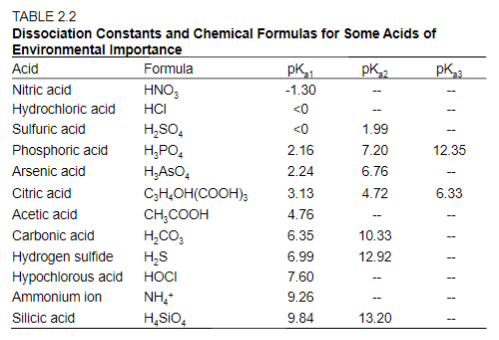
Buffer Calculations for Polyprotic Acids A polyprotic acid can form buffer solutions in presence of its conjugate base. For example, phosphoric acid can. - ppt download
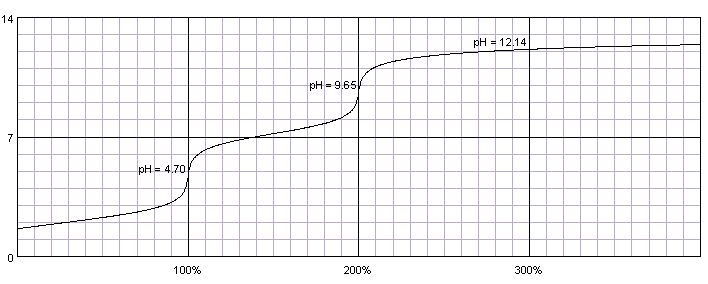
![In the acid base titration [H3PO4(0.1 M)+NaOH(0.1 M)] e.m.f of the solution is measured by coupling this electrodes with suitable reference electrode.When alkali is added pH of solution is in acoodance with In the acid base titration [H3PO4(0.1 M)+NaOH(0.1 M)] e.m.f of the solution is measured by coupling this electrodes with suitable reference electrode.When alkali is added pH of solution is in acoodance with](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/32521666_web.png)
In the acid base titration [H3PO4(0.1 M)+NaOH(0.1 M)] e.m.f of the solution is measured by coupling this electrodes with suitable reference electrode.When alkali is added pH of solution is in acoodance with

Acid-Base Test Review Definitions of Acids and Bases 1. Which of the following are Arrhenius acids? a. H2O b. H3PO4 c. NH3 d. H2SO3 2. Which of the. - ppt download
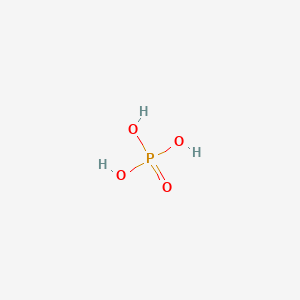
![Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid. Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid.](https://cdn1.byjus.com/wp-content/uploads/2018/11/phosphoric-acid-structure.png)
Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid.