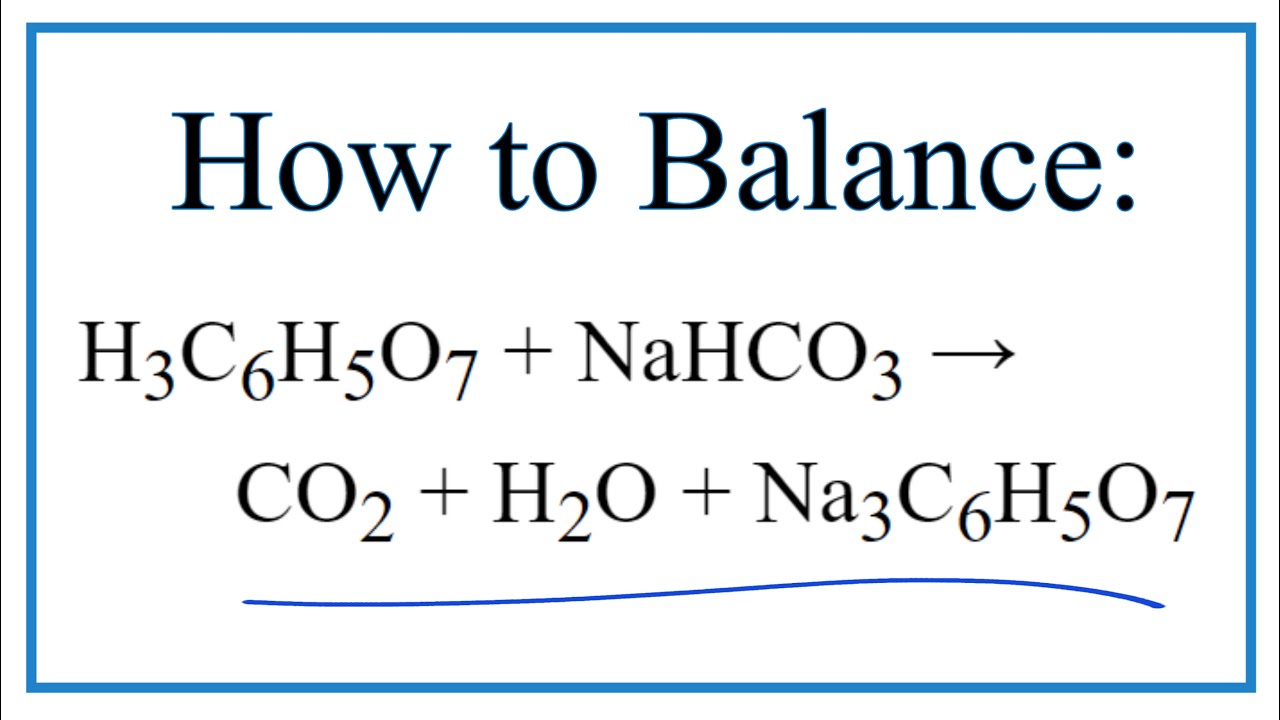
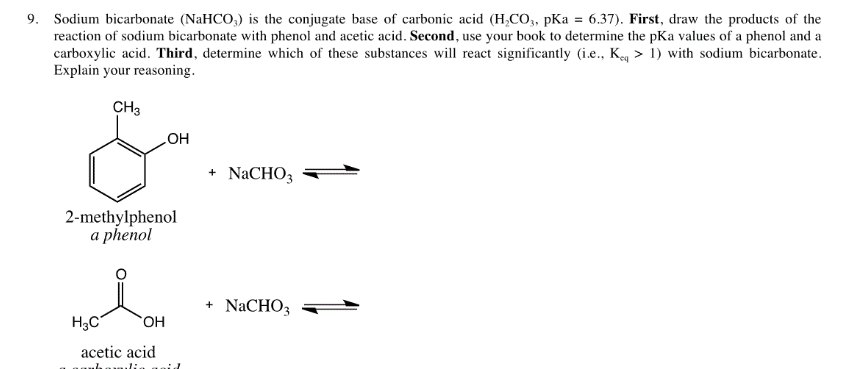
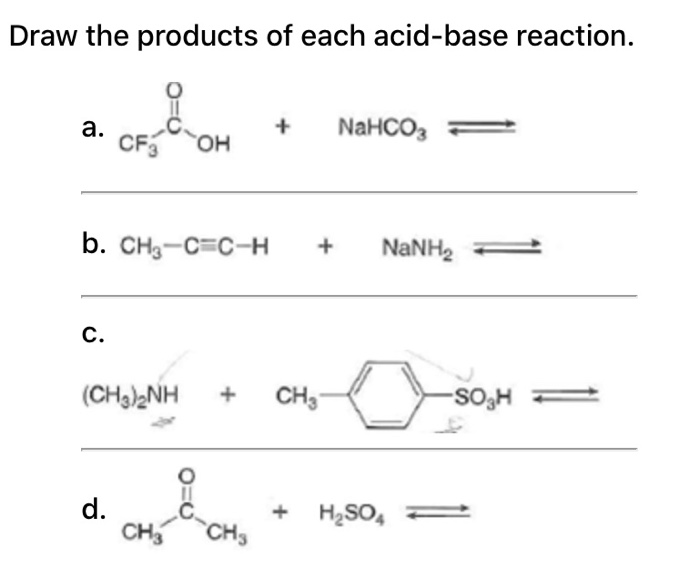
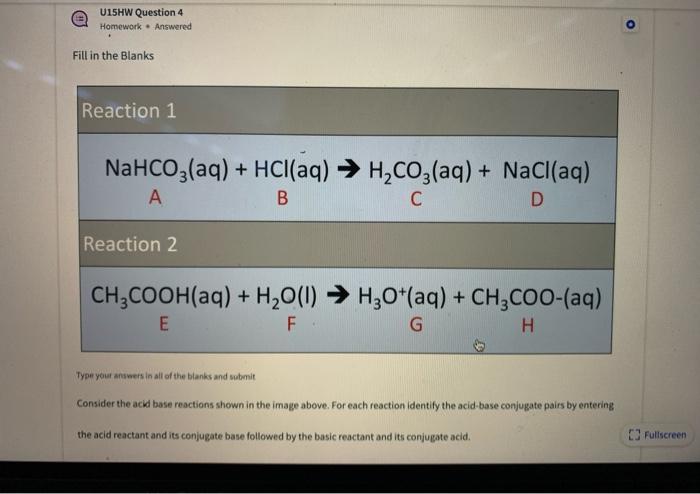
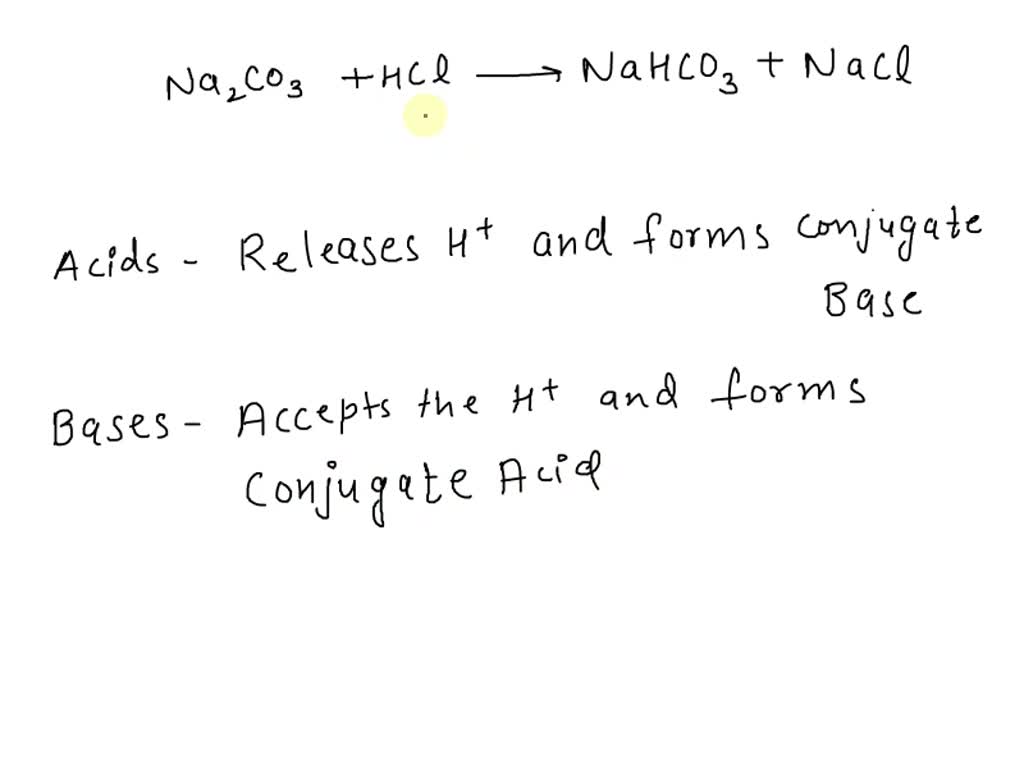
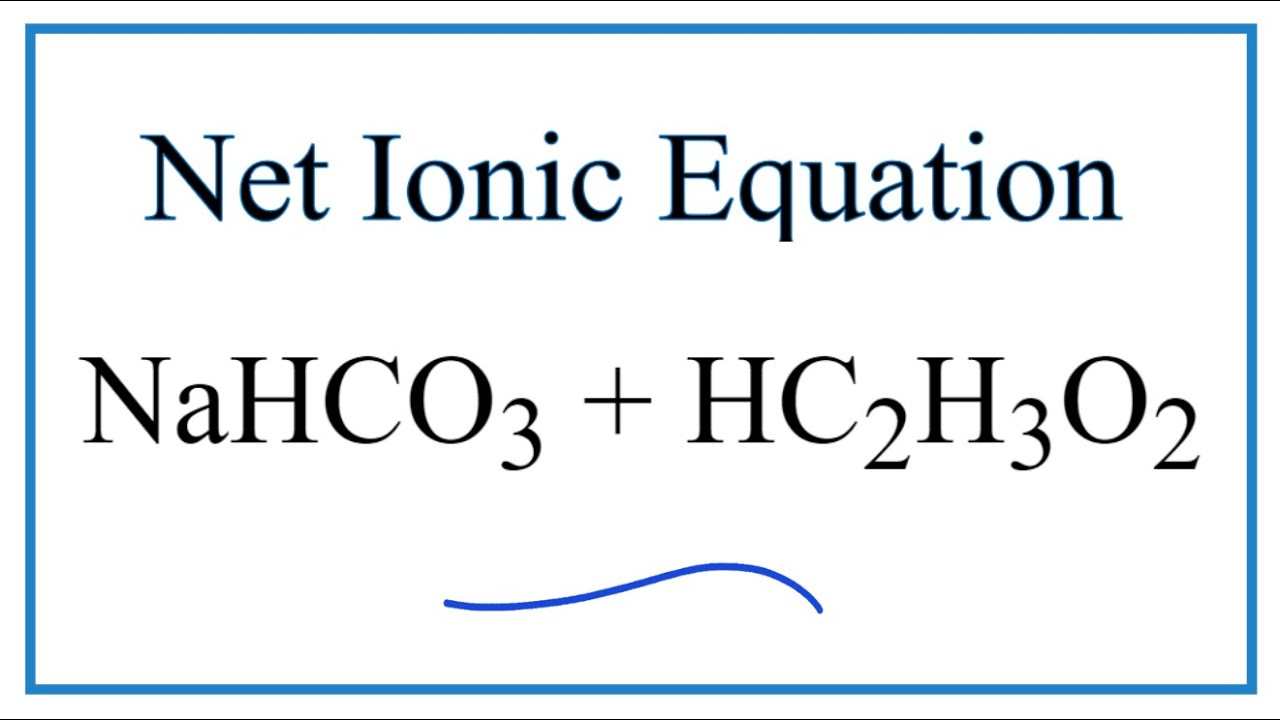How to Balance NaHCO3 + HCl = NaCl + CO2 + H2O (sodium bicarbonate plus hydrochloric acid) - YouTube

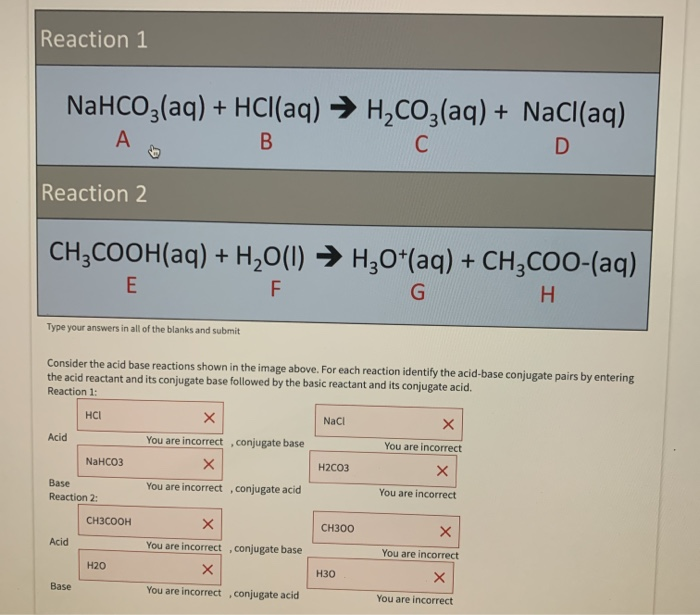
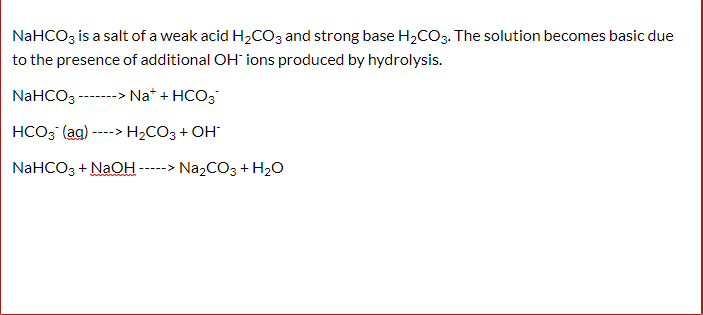
SOLVED: Consider sodium bicarbonate, NaHCOg' also known as baking soda, which is water-soluble, amphiprotic salt with K 4.8x10 and Kb 2.4x10 Which chemical equation is not something that happens when sodium bicarbonate