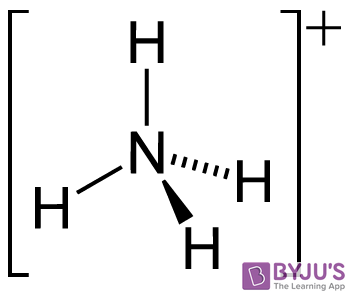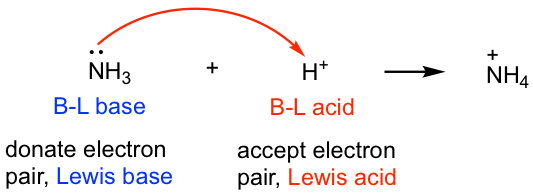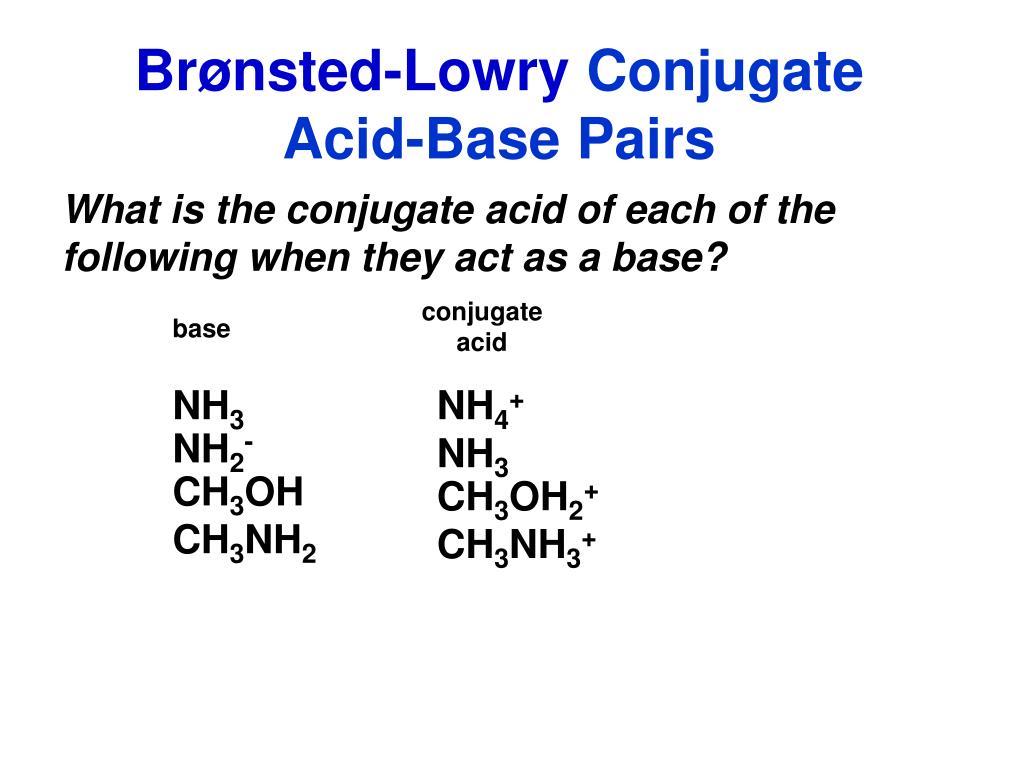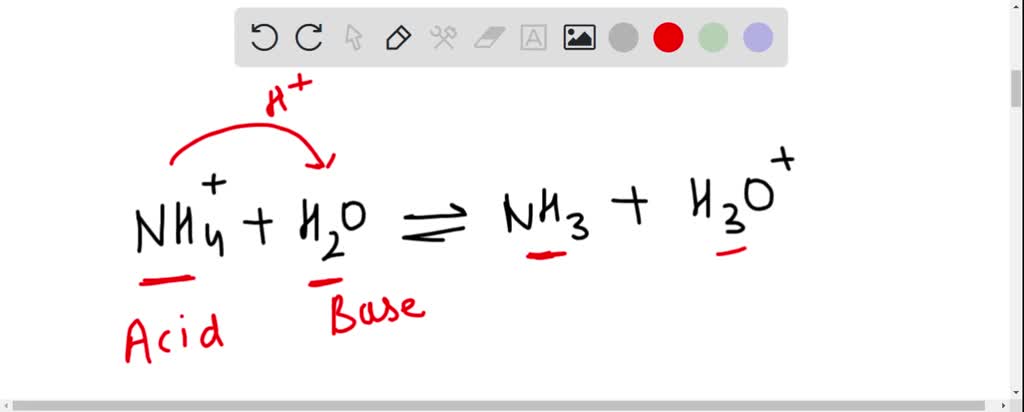
SOLVED: In the following reaction: NH4+ + H2O = NH3 + H3O+ A) H2O is a base and NH3 is its conjugate acid B) NH4+ is an acid and H20 is its

In the following acid-base reaction, HPO42- is the H2PO4- (aq) + NH3(aq) → HPO42- (aq) + - Brainly.com