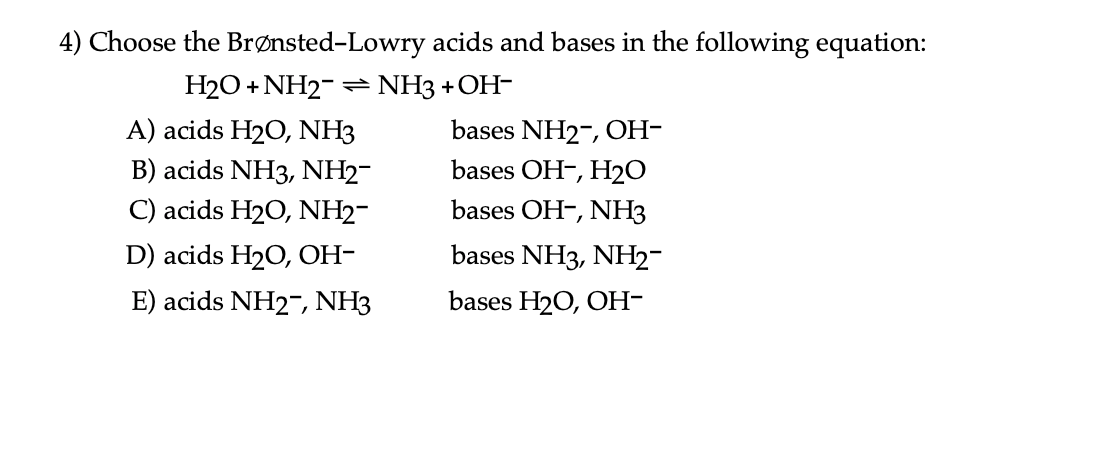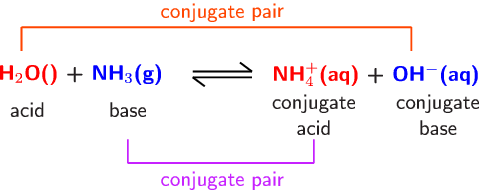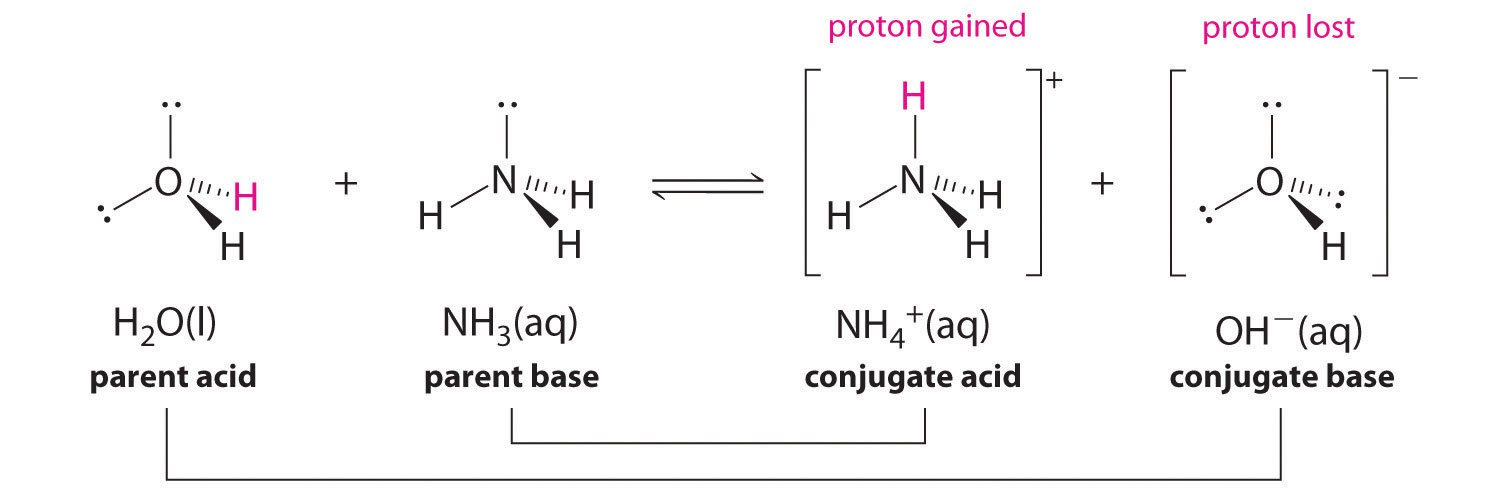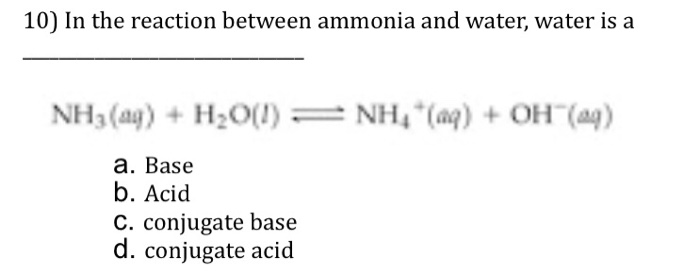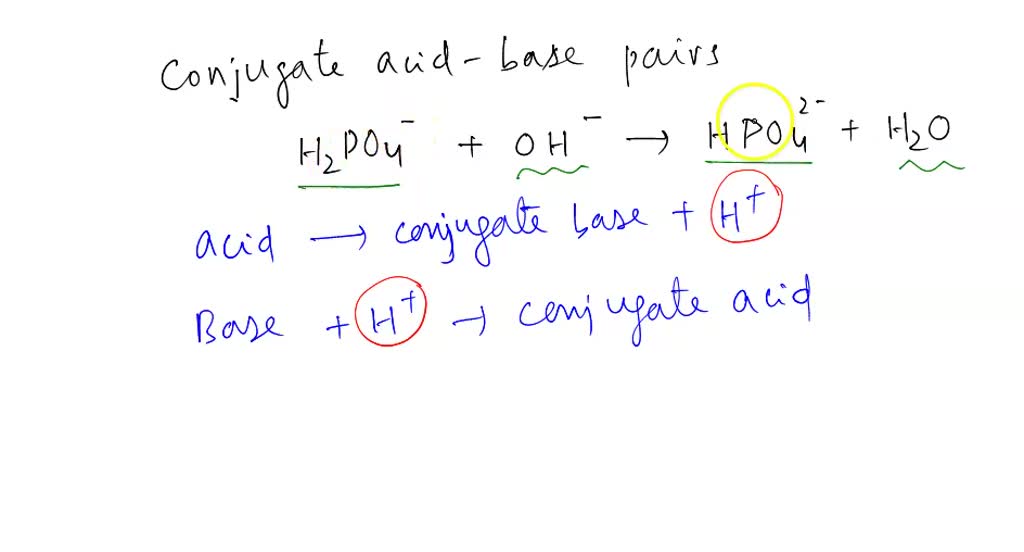
SOLVED: Determine the conjugate acid/base pairs in the following reaction: NH4+ + OH- ⇔ NH3 + H2O A. NH4+/ H2O and NH3 /OH- B. NH4+/ NH3 and OH-/H2O C. Cannot be determined.

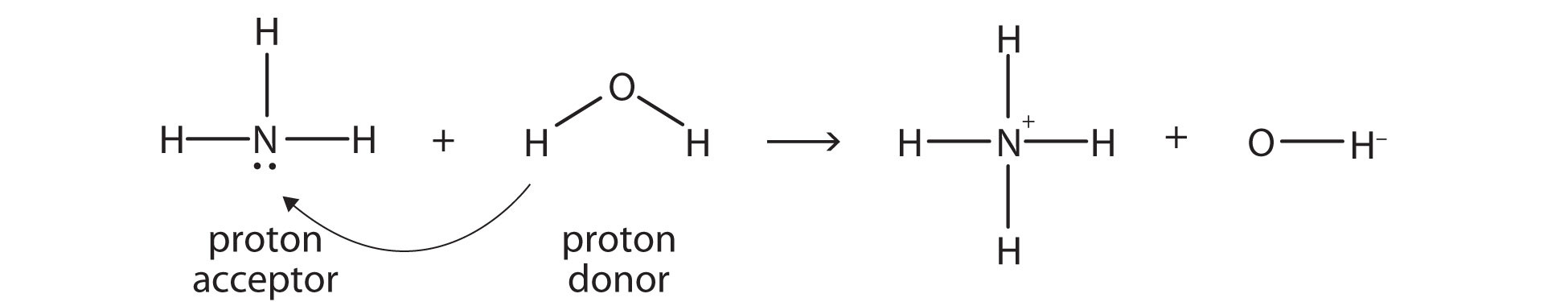
An acid-base reaction can occur when ammonia (NH3) and water (H2O) are mixed. Draw the curved arrows depicting the electron flow for the following acid- base reaction. Draw the conjugate acid and
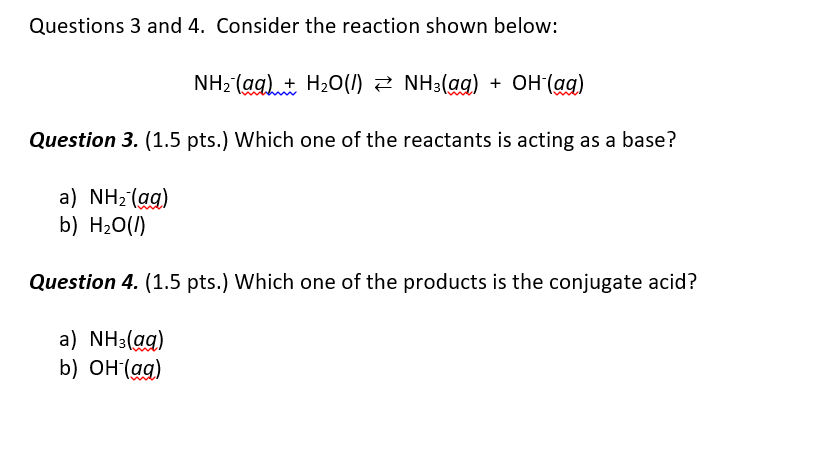
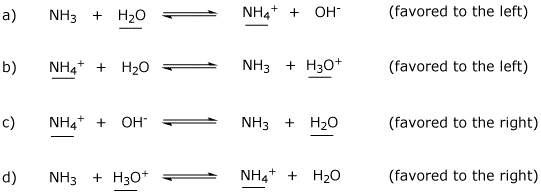
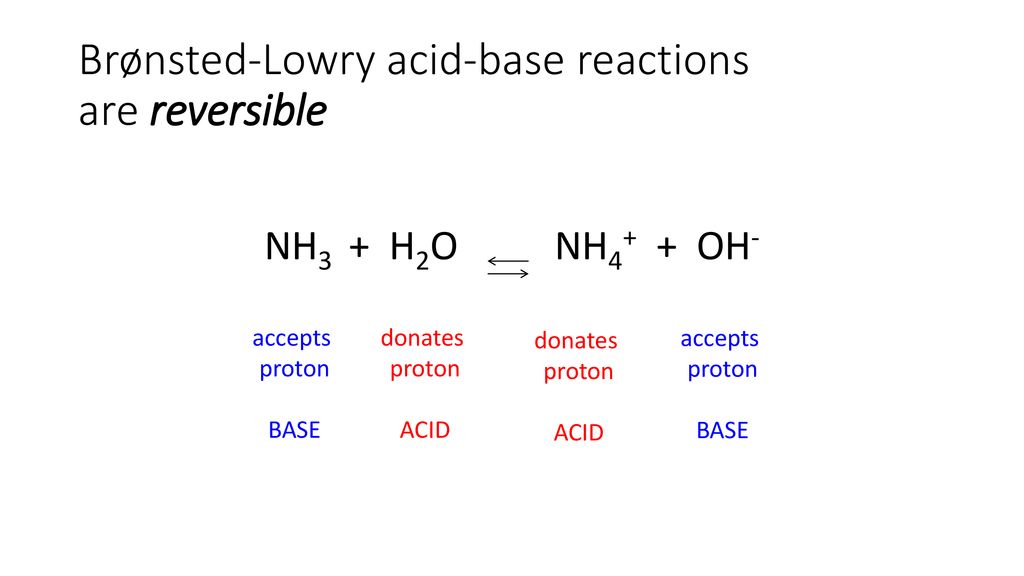
OneClass: Questions 3 and 4. Consider the reaction shown below: NH2-(ag) + H2O(l) ê·¼ NH3(gg) + OH-(a...


![The CO2-NH3-H2O system as described by the Thomsen model [7]. | Download Scientific Diagram The CO2-NH3-H2O system as described by the Thomsen model [7]. | Download Scientific Diagram](https://www.researchgate.net/publication/319196113/figure/fig1/AS:533643246669824@1504241872081/The-CO2-NH3-H2O-system-as-described-by-the-Thomsen-model-7.png)