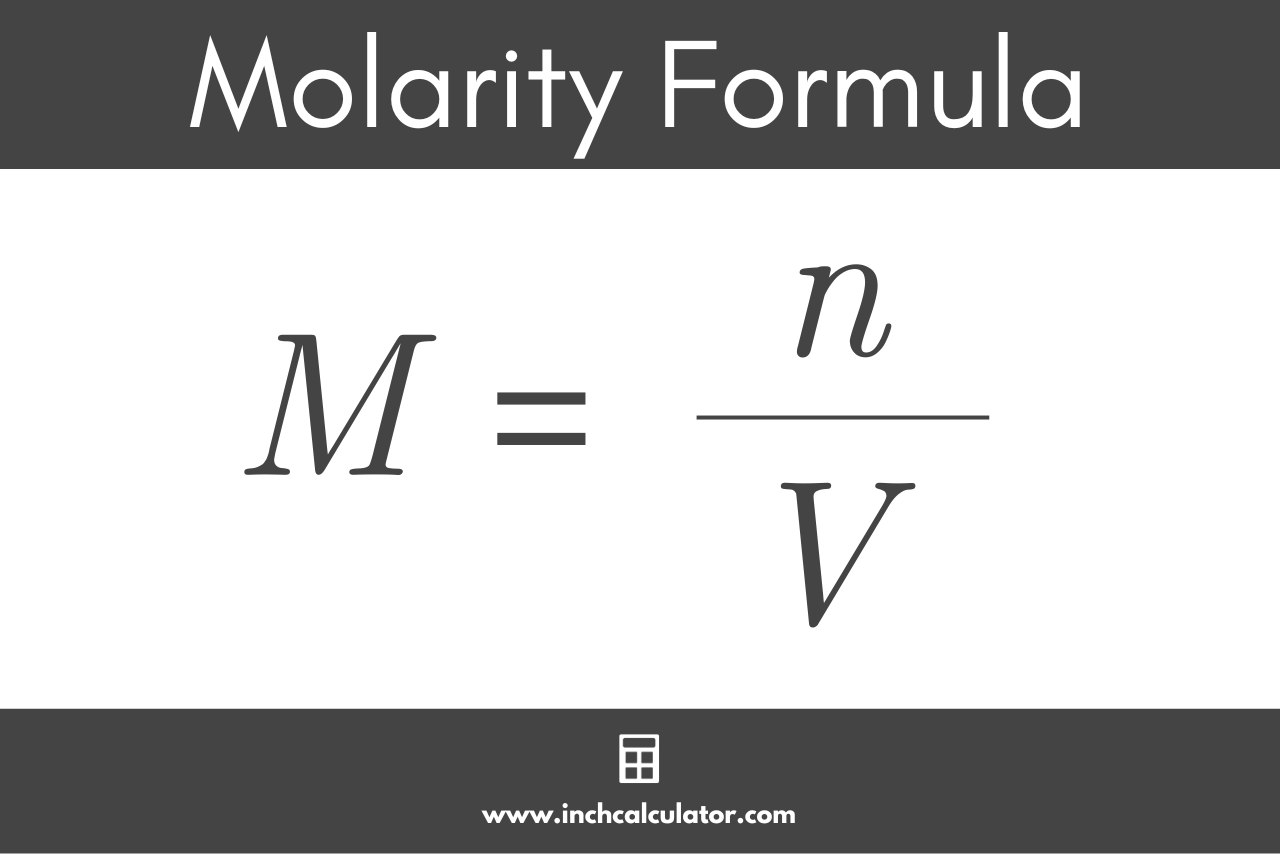I have H2O2 of molecular wt 34.01gm and 30% w/v. What does it mean that I am not getting it and I want to prepare 0.1M solution, how can i? | ResearchGate
![Calculate the molarity of 9.8 % (w/w) solution of H2SO4 if the density of the solution is 1.02 g mL^-1 .[Molar mass of H2SO4 = 98 gmol^-1] . Calculate the molarity of 9.8 % (w/w) solution of H2SO4 if the density of the solution is 1.02 g mL^-1 .[Molar mass of H2SO4 = 98 gmol^-1] .](https://dwes9vv9u0550.cloudfront.net/images/3816496/5e00d55e-bd9e-4920-bc51-c7d0813c3f94.jpg)
Calculate the molarity of 9.8 % (w/w) solution of H2SO4 if the density of the solution is 1.02 g mL^-1 .[Molar mass of H2SO4 = 98 gmol^-1] .

16 Molarity calculations ideas | converting metric units, measurement conversions, model question paper

Calculate the molality of a 20 % CaCO 3 solution by mass. The density of the solution is 1.2 g / mL.A. 1.25 mB. 2.50 mC. 1.52 mD. 2.08 m

White Board Review Practicing with Concentration Expressions Molarity Percent ppm © Mr. D. Scott; CHS. - ppt download