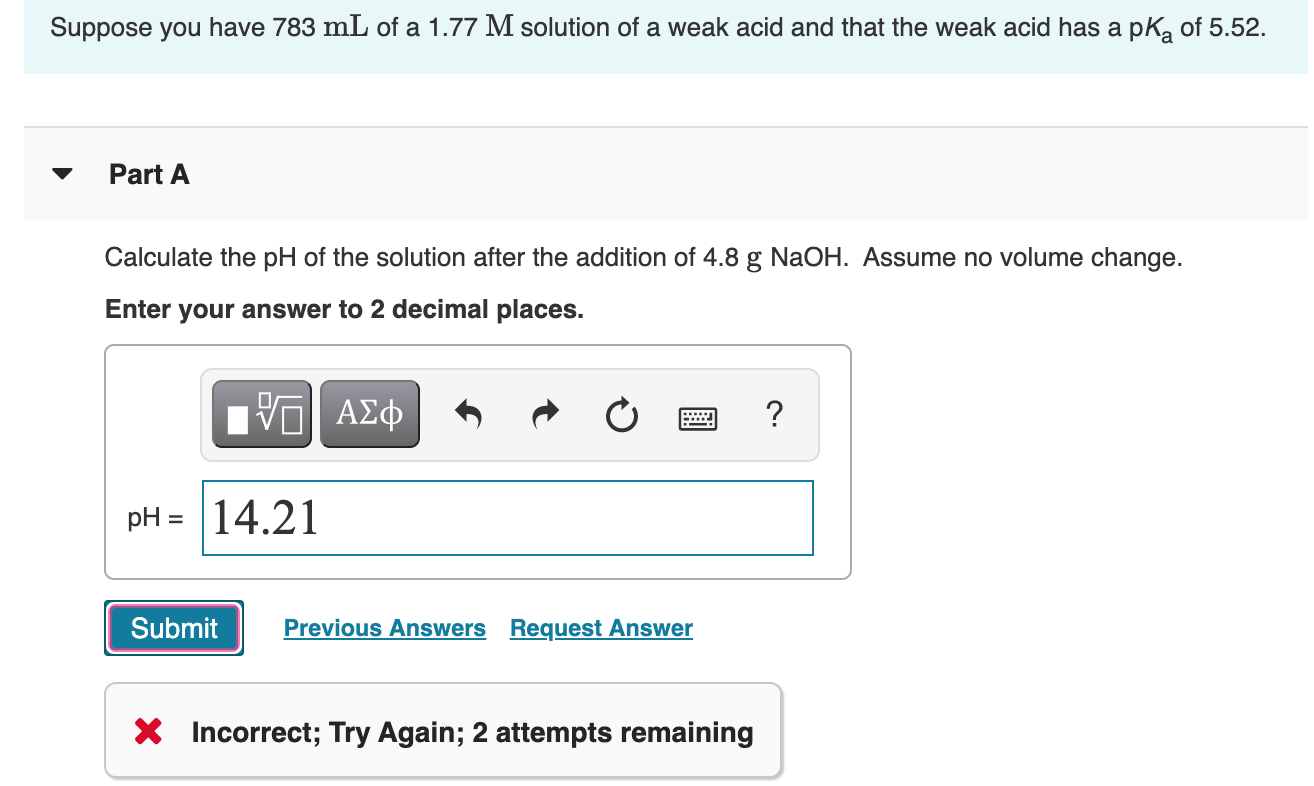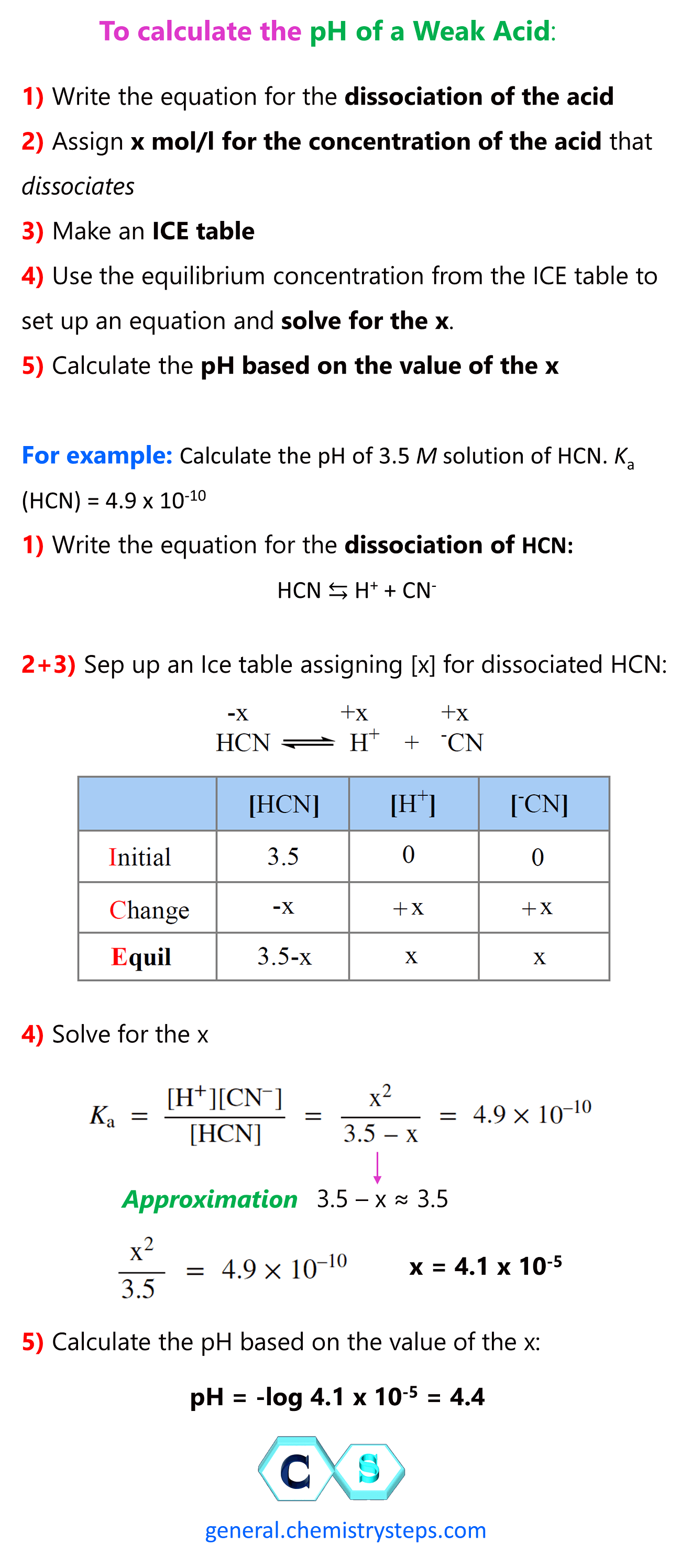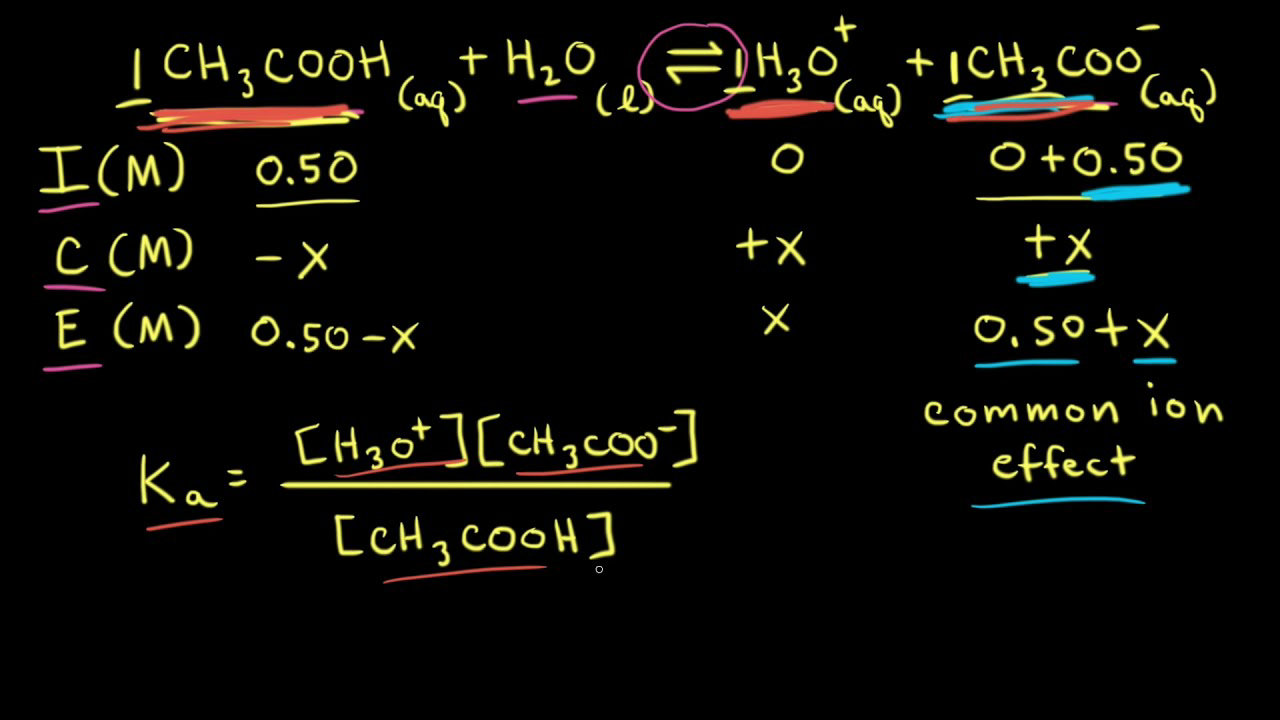
Worked example: Calculating the pH after a weak acid–strong base reaction (excess acid) (video) | Khan Academy
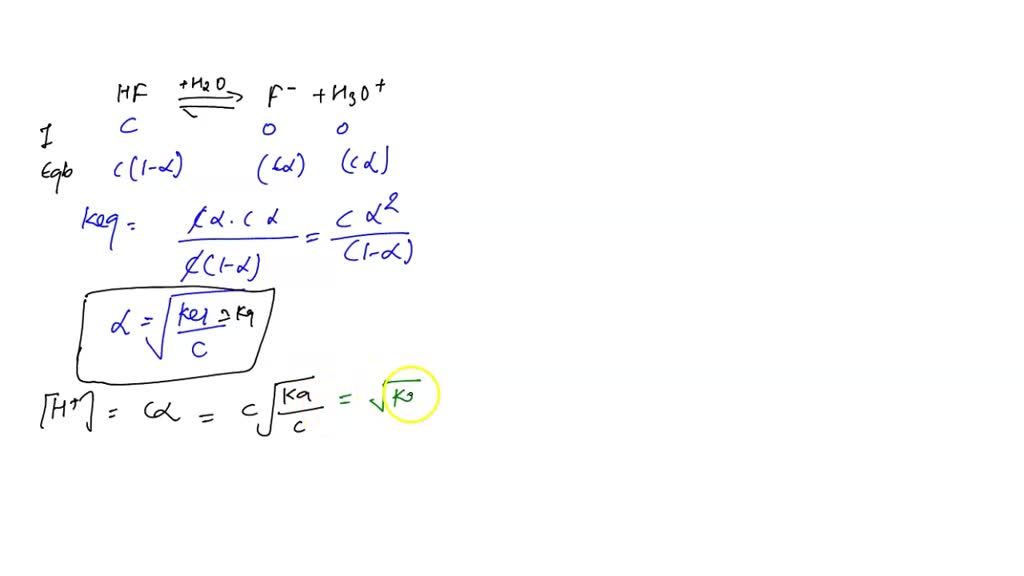
SOLVED: Calculate the pH of the weak acid HF at equilibrium, if the initial concentration of HF was 0.0340 M. (Ka = 1.45 x 10-5)
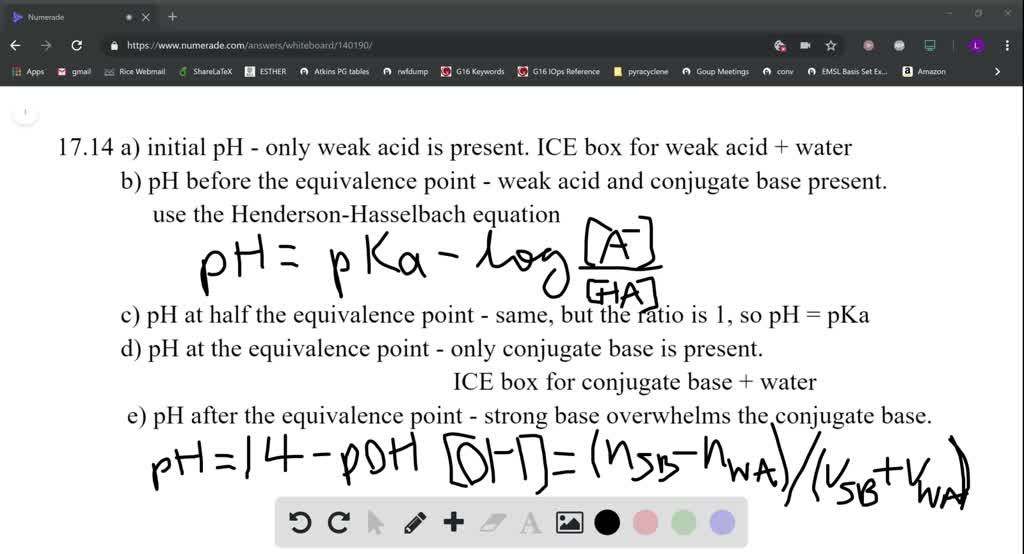
SOLVED:In the titration of a weak acid with a strong base, how do you calculate these quantities? a. initial pH b. pH before the equivalence point c. pH at one-half the equivalence